The mass
of an atom is almost entirely due to the mass of its neutrons and protons.
The mass of an electron (9.11 x 10–31 kg)
is 1/1800th of the mass of both a proton (1.673 x 10-27
kg) and a neutron (1.675 x 10-27 kg). As a result,
the mass of the electrons factors little in the the total mass of the
atom (it would take over 1800 electrons to equal the mass of 1 neutron).
The
mass of the protons + the mass of the neutrons
is an element's mass number.
Since isotopes of an element have different numbers of neutrons, they
have different mass numbers.
For example:
Two naturally occurring isotopes of chlorine are chlorine-35 & chlorine-37.
Thirty-five and thirty-seven are the mass numbers for the two isotopes.
Both isotopes have the same number of protons (17).
Isotope
name |
#
protons |
#
neutrons |
chlorine-35 |
17 |
18 |
chlorine-37 |
17 |
20 |
The number of neutrons is determined by subtracting the number of protons
from the mass number; (35-17 = 18 & 35-17 = 20 respectively).
Isotopes
are also written using their symbol with the mass number (to the upper
left) and atomic number (to the lower left).
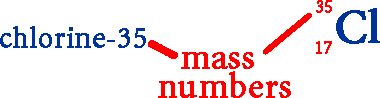
|

